By contrast far-UVC light (207 to 222 nm) has been shown to be as efficient as conventional germicidal UV light in killing microorganisms11, but studies to date12,13,14,15 suggest that these wavelengths do not cause the human health issues associated with direct exposure to conventional germicidal UV light. In short (see below) the reason is that far-UVC light has a range in biological materials of less than a few micrometers, and thus it cannot reach living human cells in the skin or eyes, being absorbed in the skin stratum corneum or the ocular tear layer. But because viruses (and bacteria) are extremely small, far-UVC light can still penetrate and kill them. Thus far-UVC light potentially has about the same highly effective germicidal properties of UV light, but without the associated human health risks12,13,14,15. Several groups have thus proposed that far-UVC light (207 or 222 nm), which can be generated using inexpensive excimer lamps, is a potential safe and efficient anti-microbial technology12,13,14,15,16,17,18 which can be deployed in occupied public locations.
The biophysically-based mechanistic basis to this far-UVC approach12 is that light in this wavelength range has a very limited penetration depth. Specifically, far-UVC light (207–222 nm) is very strongly absorbed by proteins through the peptide bond, and other biomolecules19,20, so its ability to penetrate biological materials is very limited compared with, for example, 254 nm (or higher) conventional germicidal UV light21,22. This limited penetration is still much larger than the size of viruses and bacteria, so far-UVC light is as efficient in killing these pathogens as conventional germicidal UV light12,13,14. However, unlike germicidal UV light, far-UVC light cannot penetrate either the human stratum corneum (the outer dead-cell skin layer), nor the ocular tear layer, nor even the cytoplasm of individual human cells. Thus, far-UVC light cannot reach or damage living cells in the human skin or the human eye, in contrast to the conventional germicidal UV light which can reach these sensitive cells7,8,9,10.
In summary far-UVC light is anticipated to have about the same anti-microbial properties as conventional germicidal UV light, but without producing the corresponding health effects. Should this be the case, far-UVC light has the potential to be used in occupied public settings to prevent the airborne person-to-person transmission of pathogens such as coronaviruses.
We have previously shown that a very small dose (2 mJ/cm2) of far-UVC light at 222 nm was highly efficient in inactivating aerosolized H1N1 influenza virus23. In this work we explore the efficacy of 222 nm light against two airborne human coronaviruses: alpha HCoV-229E and beta HCoV-OC43. Both were isolated over 50 years ago and are endemic to the human population, causing 15–30% of respiratory tract infections each year24. Like SARS-CoV-2, the HCoV-OC43 virus is from the beta genus25.
Here we measured the efficiency with which far-UVC light inactivates these two human coronaviruses when exposed in aerosol droplets of sizes similar to those generated during sneezing and coughing26. As all coronaviruses have comparable physical and genomic size, a critical determinant of radiation response27, we hypothesized that both viruses would respond similarly to far-UVC light, and indeed that all coronaviruses will respond similarly.
Results
Inactivation of human coronaviruses after exposure to 222 nm light in aerosols infectivity assay
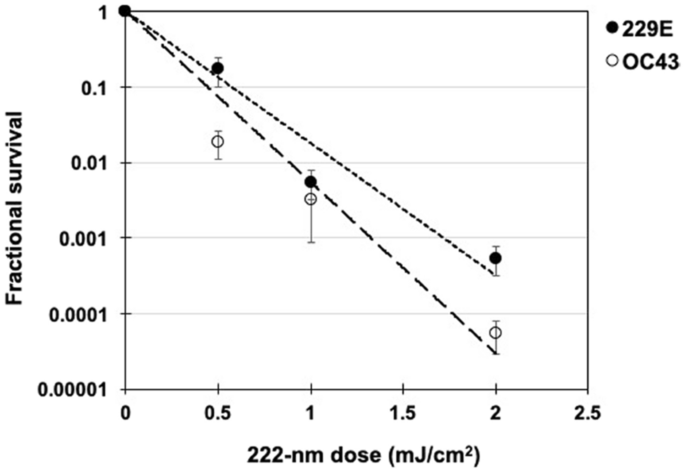
We used a standard approach to measure viral inactivation, assaying coronavirus infectivity in human host cells (normal lung cells), in this case after exposure in aerosols to different doses of far-UVC light. We quantified virus infectivity with the 50% tissue culture infectious dose TCID50assay28, and estimated the corresponding plaque forming units (PFU)/ml using the conversion PFU/ml = 0.7 TCID5029. Figure 1 shows the fractional survival of aerosolized coronaviruses HCoV-229E and HCoV-OC43 expressed as PFUUV/PFUcontrols as a function of the incident 222-nm dose. Robust linear regression (Table 1) using iterated reweighted least squares30 indicated that the survival of both genera alpha and beta is consistent with a classical exponential UV disinfection model (R2 = 0.86 for HCoV-229E and R2 = 0.78 for HCoV-OC43). For the alpha coronavirus HCoV-229E, the inactivation rate constant (susceptibility rate) was k = 4.1 cm2/mJ (95% confidence intervals (C.I.) 2.5–4.8) which corresponds to an inactivation cross-section (or the dose required to kill 90% of the exposed viruses) of D90 = 0.56 mJ/cm2. Similarly, the susceptibility rate for the beta coronavirus HCoV-OC43 was k = 5.9 cm2/mJ (95% C.I. 3.8–7.1) which corresponds to an inactivation cross section of D90 = 0.39 mJ/cm2.
Coronavirus survival as function of the dose of far-UVC light. Fractional survival, PFUUV / PFUcontrols, is plotted as a function of the 222-nm far-UVC dose. The results are reported as the estimate plaque forming units (PFU)/ml using the conversion PFU/ml = 0.7 TCID5029 by applying the Poisson distribution. Values are reported as mean ± SEM from multiple experiments (n = 3 alpha HCoV-229E and n = 4 for beta HCoV-OC43); the lines represent the best-fit regressions to equation (1) (see text and Table 1).
Viral integration assay
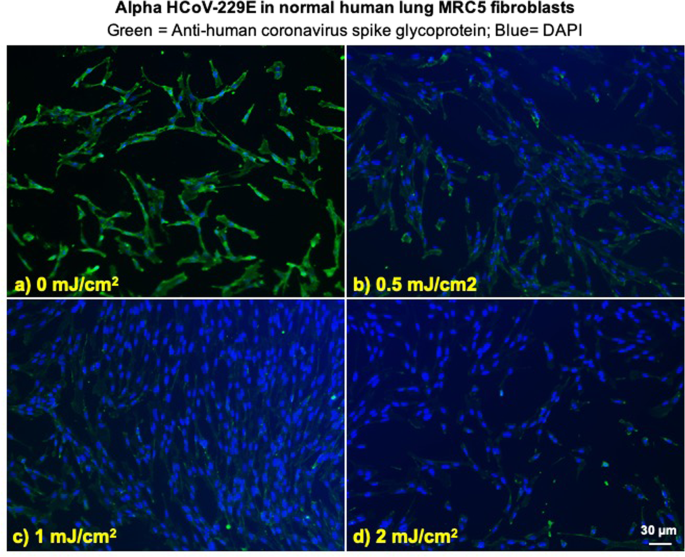
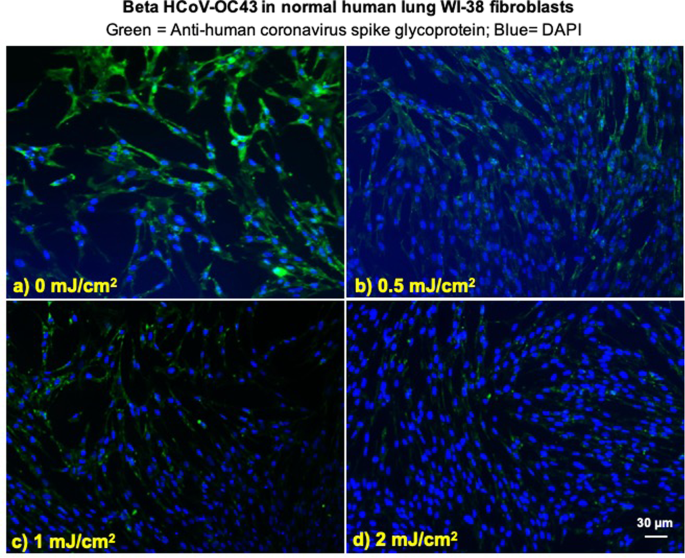
We investigated integration of the coronavirus in human lung host cells, again after exposure in aerosols to different doses of far-UVC light. Figures 2 and 3 show representative fluorescent 10x images of human lung cells MRC-5 and WI-38 incubated, respectively, with HCoV-229E (Fig. 2) and HCoV-OC43 (Fig. 3), which had been exposed in aerosolized form to different far-UVC doses. The viral solution was collected from the BioSampler after running through the aerosol chamber while being exposed to 0, 0.5, 1 or 2 mJ/cm2 of 222-nm light. Cells were incubated with the exposed virus for one hour, the medium was replaced with fresh infection medium, and immunofluorescence was performed 24 hours later. We assessed the human cell lines for expression of the viral spike glycoprotein, whose functional subunit S2 is highly conserved among coronaviruses31,32. In Figs. 2 and 3, green fluorescence (Alexa Fluor-488 used as secondary antibody against anti-human coronavirus spike glycoprotein antibody) qualitatively indicates infection of cells with live virus, while the nuclei were counterstained with DAPI appearing as blue fluorescence. For both alpha HCoV-229E and beta HCoV-OC43, exposure to 222-nm light reduced the expression of the viral spike glycoprotein as indicated by a reduction in green fluorescence.
Infection of human lung cells from irradiated aerosolized alpha HCoV-229E as function of dose of far-UVC light. Representative fluorescent images of MRC-5 normal human lung fibroblasts infected with human alphacoronavirus 229E exposed in aerosolized form. The viral solution was collected from the BioSampler after running through the aerosol chamber while being exposed to (a) 0, (b) 0.5, (c) 1 or (d) 2 mJ/cm2 of 222-nm light. Green fluorescence qualitatively indicates infected cells (Green = Alexa Fluor-488 used as secondary antibody against anti-human coronavirus spike glycoprotein antibody; Blue = nuclear stain DAPI). Images were acquired with a 10× objective; the scale bar applies to all the panels in the figure.
Infection of human lung cells from irradiated aerosolized beta HCoV-OC43 as function of dose of far-UVC light. Representative fluorescent images of WI-38 normal human lung fibroblasts infected with human betacoronavirus OC43 exposed in aerosolized form. The viral solution was collected from the BioSampler after running through the aerosol chamber while being exposed to (a) 0, (b) 0.5, (c) 1 or (d) 2 mJ/cm2 of 222-nm light. Green fluorescence qualitatively indicates infected cells (Green = Alexa Fluor-488 used as secondary antibody against anti-human coronavirus spike glycoprotein antibody; Blue = nuclear stain DAPI). Images were acquired with a 10× objective; the scale bar applies to all the panels in the figure.
Discussion
The severity of the 2020 COVID-19 pandemic warrants the rapid development and deployment of effective countermeasures to reduce indoor person-to-person transmission. We have developed a promising approach using single-wavelength far-UVC light at 222 nm generated by filtered excimer lamps, which inactivates airborne viruses without inducing biological damage in exposed human cells and tissue11,12,13,14,15,16,17,18. The approach is based on the biophysically-based principle that far-UVC light, because of its very limited penetration in biological materials, can traverse and kill viruses and bacteria which are typically micrometer dimensions or smaller, but it cannot penetrate even the outer dead-cell layers of human skin, nor the outer tear layer on the surface of the human eye12.
In this work we have used an aerosol irradiation chamber to test the efficacy of 222-nm far-UVC light to inactivate two aerosolized human coronaviruses, beta HCoV-OC43 and alpha HCoV-229E. As shown in Fig. 1, inactivation of the two human coronavirus by 222-nm light follows a typical exponential disinfection model, with an inactivation constant for HCoV-229E of k = 4.1 cm2/mJ (95% C.I. 2.5–4.8), and k = 5.9 cm2/mJ (95% C.I. 3.8–7.1) for HCoV-OC43. These values imply that 222 nm UV light doses of only 1.7 mJ/cm2 or 1.2 mJ/cm2 respectively produce 99.9% inactivation (3-log reduction) of aerosolized alpha HCoV-229E or beta HCoV-OC43. A summary of k values and the corresponding D90, D99, and D99.9 values we have obtained for coronaviruses is shown in Table 2, together with our earlier results for aerosolized H1N1 influenza virus23. The relatively small difference in influenza A (H1N1) and human coronaviruses sensitivity to 222-nm light is likely attributable to differences in structure, genome size, and nucleic acid configuration33. It is also important to note that the previous results with H1N1 virus utilized a fluorescent focus assay to assess virus survival23 in contrast to this work which used the TCID50 assay. While both assays are widely used to accurately determine viral infectivity34, the former employs immunofluorescence to detect a specific viral antigen, instead of depending on cytopathic effects as in the TCID50 assay. Because the assays differ in methods and principles, some variance is expected between these two techniques.
The results suggest that both of the studied coronavirus strains have similar high sensitivity to far-UVC inactivation. Robust linear regression produced overlapping 95% confidence intervals for the inactivation rate constant, k, of 2.5 to 4.8 cm2/mJ and 3.8 to 7.1 cm2/mJ respectively for the 229E and OC43 strains. As all human coronaviruses have similar genomic sizes which is a primary determinant of UV sensitivity27, it is reasonable to expect that far-UVC light will show similar inactivation efficiency against all human coronaviruses, including SARS-CoV-2. The data obtained here are consistent with this hypothesis.
It is useful to compare the performance of far-UVC light with conventional germicidal (peak 254 nm) UVC exposure. We are aware of only one such study35, which used an aerosolized murine beta coronavirus. The study reported a D88 of 0.599 mJ/cm2, which others4 have used to estimate the D90 for the virus with 254 nm light as 0.6 mJ/cm2. This value is similar to those estimated in the current work (see Table 2), suggesting similar inactivation efficiency of 222 nm far-UVC and conventional germicidal 254 nm UVC for aerosolized coronavirus, and providing further support for the suggestion that all coronaviruses have similar sensitivities to UV light.
The sensitivity of the coronaviruses to far-UVC light, together with extensive safety data even at much higher far-UVC exposures12,13,14,15,16,17,18, suggests that it may be feasible and safe to have the lamps providing continuous low-dose far-UVC exposure in public places – potentially reducing the probability of person-to-person transmission of coronavirus as well as other seasonal viruses such as influenza. In fact there is a regulatory limit as to the amount of 222 nm light to which the public can be exposed, which is 23 mJ/cm2 per 8-hour exposure36,37. Based on our results here for the beta HCoV-OC43 coronavirus, continuous far-UVC exposure at this regulatory limit would result in 90% viral inactivation in approximately 8 minutes, 95% viral inactivation in approximately 11 minutes, 99% inactivation in approximately 16 minutes and 99.9% inactivation in approximately 25 minutes. Thus continuous airborne disinfection with far-UVC light at the currently regulatory limit would provide a major reduction in the ambient level of airborne virus in occupied indoor environments.
In conclusion, we have shown that very low doses of far-UVC light efficiently kill airborne human coronaviruses carried by aerosols. A dose as low as 1.2 to 1.7 mJ/cm2 of 222-nm light inactivates 99.9% of the airborne human coronavirus tested from both genera beta and alpha, respectively. As all human coronaviruses have similar genomic size, a key determinant of radiation sensitivity27, it is likely that far-UVC light will show comparable inactivation efficiency against other human coronaviruses, including SARS-CoV-2.
Together with previous safety studies12,13,14,15,16,17,18 and our earlier studies with aerosolized influenza A (H1N1)23, these results suggest the utility of continuous low-dose-rate far-UVC light in occupied indoor public locations such as hospitals, transportation vehicles, restaurants, airports and schools, potentially representing a safe and inexpensive tool to reduce the spread of airborne-mediated viruses. While staying within the current regulatory dose limits, low-dose-rate far-UVC exposure can potentially safely provide a major reduction in the ambient level of airborne coronaviruses including SARS-CoV-2.
Methods
Viral strains
HCoV-229E (VR-740) and HCoV-OC43 (VR-1558) were propagated in human diploid lung MRC-5 fibroblasts (CCL-171) and WI-38 (CCL-75), respectively (all from ATCC, Manassas, VA). Both human cell lines were grown in MEM supplemented with 10% Fetal Bovine Serum (FBS), 2 mM L-alanyl-L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich Corp. St. Louis, MO, USA). The virus infection medium consisted of MEM or RPMI-1640 plus 2% heat inactivated FBS for HCoV-229E and HCoV-OC43, respectively. The viral strains were propagated by inoculation of flasks containing 24-hours old host cells, which were 80–90% confluent. After one hour incubation, the cell monolayer was washed and incubated in fresh infection medium for three or four days at 35 °C for HCoV-229E and at 33 °C for HCoV-OC43. The supernatant containing the working viral stock was then collected by centrifugation (300 g for 15 minutes). The virus titer was determined by 50% tissue culture infective dose TCID50 by assessing cytopathic effects (CPE), which were scored at a bright field microscope (10×) as vacuolization of cytoplasm, cell rounding and sloughing.
Benchtop aerosol irradiation chamber
A one-pass, dynamic aerosol/virus irradiation chamber was used to generate, expose, and collect aerosol samples as previously described23. Viral aerosols were generated by adding a virus solution in a high-output extended aerosol respiratory therapy nebulizer (Westmed, Tucson, AZ) and operating using an air pump with an input flow rate of 11 L/min. Virus flowed into the chamber and was mixed with dry and humidified air to maintain humidity between approximately 50–70%. The relative humidity, temperature, and aerosol particle size distribution were monitored throughout operation. Aerosol was exposed to far-UVC light and finally collected using a BioSampler (SKC Inc., Eighty Four, PA).
The far-UVC lamp was positioned approximately 22 cm away from the UV exposure chamber and directed at the 26 cm × 25.6 cm × 254 μm UV-transmitting plastic window (TOPAS 8007 × 10, TOPAS Advanced Polymers Inc., Florence, KY). Consistent with our previous experiments using this chamber23, the flow rate through the system was 12.5 L/min. The volume of the UV exposure region was 4.2 L so each aerosol was exposed for approximately 20 seconds as it traversed the window. The entire irradiation chamber was contained in a biosafety level 2 cabinet and all air inputs and outputs were equipped with HEPA filters (GE Healthcare Bio-Sciences, Pittsburgh, PA) to prevent unwanted contamination from entering or exiting the system.
Irradiation chamber performance
The custom irradiation chamber simulated the transmission of aerosolized viruses produced via human coughing and breathing. The chamber operated at an average relative humidity of 66% and an average temperature of 24 °C across all runs. The average particle size distribution was 83% between 0.3 μm and 0.5 μm, 12% between 0.5 μm and 0.7 μm, and 5% >0.7 μm (Table 3). Aerosolized viruses were efficiently transmitted through the system as evidenced from the control (zero exposure) showing clear virus integration (Figs. 2 and 3, top left panels).